I-Sugammadex Sodium
| 舒更葡糖钠 | I-Sugammadex Sodium | 343306-79-6 | Endlini |
Incazelo
I-Sugammadex sodium iyi-synthetic γ-cyclodextrin derivative, futhi isebenza njenge-ejenti entsha yokuhlehla ye-neuromuscular block.
Igama elijwayelekile: sugammadex (soo GAM ma dex)
Igama lebrand: Bridion
I-Sugammadex ibuyisela emuva imiphumela yemithi ethile enikezwa ngesikhathi sokuhlinzwa ukuze uphumule imisipha yakho.
I-Sugammadex isetshenziswa ekupheleni kokuhlinzwa, ukusiza ukubuyisela ukusebenza kwemisipha okuye kwavinjelwa ngesikhathi sokuhlinzwa eminye imithi.
I-Sugammadex ingase isetshenziselwe izinjongo ezingabaliwe kulo mhlahlandlela wemithi.
Isetshenziselwa ukubuyisela emuva imiphumela yezinye izidakamizwa.
Ku-Vivo
Umjovo we-sugammadex awunayo imiphumela ebalulekile kumfutho wegazi noma ukushaya kwenhliziyo.Ngemuva kokujova umthamo ophezulu we-rocuronium, ukubuyiselwa kwe-90% kwesilinganiso sesitimela-sesine kuthatha i-28 min (SD 7 min) ngemva kwe-saline, i-26 min (SD 9.5 min) ngemva kwe-1 mg / kg sugammadex, ne-8 min (SD 3.6 min) ngemva kwe-2.5 mg/kg sugammadex[1].I-Sugammadex ibangela ukuguqulwa okusheshayo nokuphelele kwe-rocuronium-induced neuromuscular block.Isikhathi sokutakula sokuqeqesha okune isilinganiso=0.9 ngemva kokululama okuzenzakalelayo yimizuzu engu-14.4 (SD=3.4 min; n=14).Lokhu kuncishiswe kakhulu kumaminithi angu-3.7 (SD=3.3 min; n=4) nge-sugammadex 0.5 mg/kg kanye namaminithi angu-1.9 (SD=1.0 min; n=4) nge-sugammadex 1.0 mg/kg[2].Isilinganiso sempilo yesigamu se-sugammadex ku-Rhesus monkey singama-30 (SEM=4.9) amaminithi[3].
Isitoreji
| Impushana | -20°C | 3 iminyaka |
| 4°C | iminyaka engu-2 | |
| Ku-solvent | -80°C | 6 izinyanga |
| -20°C | Inyanga engu-1 |
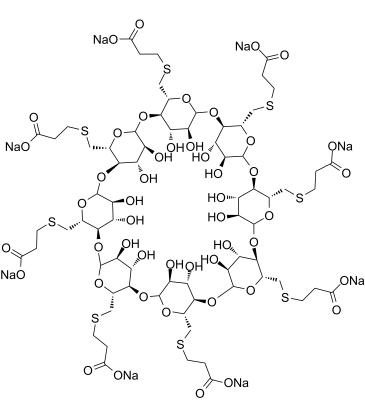
Isakhiwo samakhemikhali





Isiphakamiso18Amaphrojekthi Wokuhlola Ukuvumelana Kwekhwalithi asegunyaziwe4, futhi6amaphrojekthi angaphansi kokugunyazwa.

Uhlelo lokuphatha ikhwalithi yamazwe ngamazwe oluthuthukile lwenze isisekelo esiqinile sokuthengisa.

Ukugadwa kwekhwalithi kusebenza kuwo wonke umjikelezo wempilo womkhiqizo ukuze kuqinisekiswe ikhwalithi nomphumela wokwelapha.

Ithimba Lezindaba Zokulawula Ochwepheshe lisekela izimfuno zekhwalithi ngesikhathi sokufaka isicelo nokubhaliswa.


I-Korea Countec Bottled Packaging Line


I-Taiwan CVC Bottled Packaging Line


I-Italy CAM Board Packaging Line

Umshini Wokuhlanganisa we-Fette waseJalimane

I-Japan Viswill Tablet Detector

I-DCS Control Room










